The Impact of Toxic Heavy Metals on the Hypothalamic-Pituitary-Adrenal (HPA) Axis
Toxic heavy metals are everywhere. Arsenic is in rice crackers. Mercury is in sushi. Baby food might contain lead, cadmium, and other toxic metals. Your favorite dark chocolate could also be contaminated with cadmium and lead!
Unfortunately, everyone, including your patients, has toxic metals in their body, and the more toxic heavy metals present, the higher the risk of developing many health concerns, including cancer, cardiovascular disease, infertility, and diabetes. Research shows exposure to toxic heavy metals can also impact the health of the Hypothalamic-Pituitary-Adrenal (HPA) axis, the production of cortisol (the stress hormone), and the production of other hormones.
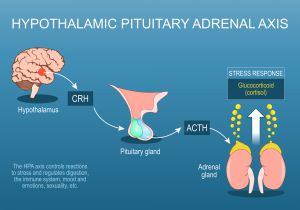
What is the Hypothalamic-Pituitary-Adrenal (HPA) Axis?
The communication between the brain and the adrenal glands is known as the hypothalamic-pituitary-adrenal axis – or the HPA axis. The adrenal glands produce hormones, including cortisol, DHEA, and progesterone, in response to signals from the brain and play a primary role in maintaining a healthy circadian rhythm and an optimal stress response.
 How Do Toxic Heavy Metals Harm the Hypothalamic – Pituitary – Adrenal (HPA) Axis?
How Do Toxic Heavy Metals Harm the Hypothalamic – Pituitary – Adrenal (HPA) Axis?
A Brief Summary
Some of the mechanisms by which toxic metals may harm the HPA axis include:
- Increased oxidative stress
- Abnormal enzyme activity
- Reduction in cofactors available for optimal enzyme activity
- Altered metabolism of hormones
- A change in the number of hormone receptors
- Altered hormone receptor function
- Induction of autoimmune activity1
- Inhibition of hormone production
- Impairment of signaling from the hypothalamus and pituitary2
- Mitochondrial dysfunction1,3
 The Toxic Effects of the Metalloestrogen Cadmium on the HPA Axis
The Toxic Effects of the Metalloestrogen Cadmium on the HPA Axis
Research shows several toxic metals can harm the HPA axis. One toxic metal that significantly impacts the function of the HPA axis and cortisol production is cadmium. According to some researchers, the hypothalamic-pituitary axis could be the primary target of cadmium toxicity since cadmium accumulates in the hypothalamus and pituitary gland.4
Cadmium is a persistent contaminant and was one of the most commonly used heavy metals in the 1970s. Cadmium compounds are used in pigments, plastics, radiation screens, rechargeable batteries, fertilizers, and more. It has also been used as a protective coating on steel and in alloys.4
Research suggests cadmium exposure can contribute to changes in pituitary hormone production, inhibit progesterone synthesis in vivo and in vitro, stimulate estrogenic effects as a metalloestrogen, alter gonadal development, cause gonadal damage, and have harmful effects on sperm.4,5 Cadmium administration reduces membrane fluidity in the pituitary gland, which could be one mechanism by which cadmium exposure affects receptor function and the secretion of pituitary hormones.4
Concerning the HPA axis, exposure to cadmium alters the secretion of adrenocorticotropin hormone (ACTH), and the effects are dose-dependent.4,6 In vivo studies also show cadmium exposure leads to higher TSH levels and lower T3 and T4 levels, which could also impact the overall function of the HPA axis.6
The data suggest cadmium exposure may either increase or decrease cortisol levels, depending on the duration and magnitude of the toxic exposure.7
 The Toxic Effects of Lead on the HPA Axis and Cortisol Production
The Toxic Effects of Lead on the HPA Axis and Cortisol Production
Lead has historically been and is currently present in paints, pesticides, gasoline, coatings, glass, batteries, and plumbing fixtures. Although the production of leaded gasoline and paints has been prohibited, lead is still abundant in the environment. Research suggests that there is no safe level of lead in humans since lead is an endocrine-disrupting, non-degradable toxic substance that can cause irreversible health damage.8
Lead exposure is significantly associated with significant alterations in cognitive and behavioral scores, IQ, neurotransmitters, and inflammatory cytokine levels in children. Regarding the endocrine system, exposure to lead is positively associated with the production of Sex Hormone Binding Globulin (SHBG), which could reduce the levels of bioactive, free steroid hormones, including testosterone.8
Research also shows adults with lead poisoning may experience changes in thyroid function. Those with higher lead levels are at a higher risk of developing hyperthyroidism, characterized by low TSH and high thyroxine (T4) levels, than controls. Lead exposure can damage multiple systems in the body, and the endocrine system is a significant target. The deleterious effects of lead on the HPA axis and cortisol production have also been studied.8
Research suggests exposure to toxic lead may affect the basal, or baseline, cortisol level. Studies also indicate exposure to lead contributes to a stunted stress response, which may be a factor in the development of learning impairments.9
The results of animal studies suggest maternal lead exposure can permanently alter the responsivity to stress challenges and HPA axis function in their offspring. Animals exposed to lead experienced decreased basal plasma corticosterone levels and reduced glucocorticoid receptor binding. Responsivity to cold stress markedly increased corticosterone production only in animals treated with lead.10
Like cadmium, lead administration lowers membrane fluidity in the pituitary gland, which may impact receptor binding and the secretion of pituitary hormones.4,11
 The Toxic Effects of the Endocrine Disruptor Mercury on the HPA Axis
The Toxic Effects of the Endocrine Disruptor Mercury on the HPA Axis
Mercury exposure can occur due to natural phenomena and human behavior. Natural sources of mercury include geologic deposits of mercury, volcanoes, and volatilization from the ocean. Sources of mercury from human activity include the release of mercury during alkali and metal processing, incineration of coal, mining of gold and mercury, and disposal of medical and other waste.12
Most individuals who experience occupational mercury exposure work in the chlor-alkali industry or as laboratory technicians, goldsmiths, gold miners, dental technicians, or dentists. Non-occupational exposure to mercury is primarily from dental amalgams, ophthalmological solutions, vaccines, skin-lightening creams, folk medicines, and consumption of fish and shellfish.12
According to animal studies, mercury exposure could alter the structure of the adrenal glands, leading to enlargement.13 Thus far, the research on the effects of mercury exposure on hormone production conflicts.
One animal study determined exposure to mercury increased plasma cortisol levels but decreased plasma testosterone levels in rainbow trout. In catfish, mercury exposure significantly lowers plasma cortisol levels, while yellow perch and northern pike exposed to mercury demonstrate an impaired cortisol response to the acute stress of capture.12
A similar pattern of conflicting results – mercury exposure inducing increased and decreased plasma cortisol levels – is observed in rats. One study reported that mercury exposure led to a 50% reduction in corticosterone production, a 300% increase in plasma DHEA (dehydroepiandrosterone), and a 70% increase in plasma progesterone in rats. Some animal studies suggest no change in cortisol levels during a stress response after mercury exposure.12 More research is needed.
The results of a clinical study suggest pregnant women with higher mercury levels have a blunted morning cortisol response.14 An inverse association between inorganic mercury concentrations in the blood and levels of Luteinizing Hormone (LH), a pituitary hormone, has also been noted in a clinical study.15
 Research suggests mercury may affect the HPA axis by:
Research suggests mercury may affect the HPA axis by:
- Altering the neuroendocrine regulation of several hormones released by the pituitary gland, including prolactin, gonadotropin, and melanocyte-stimulating hormone.
- Interfering with the optimal function of the cells that synthesize hormones in the pituitary gland.
- Directly affecting the release of ACTH from the pituitary gland and the downstream production of corticosteroids by the adrenal glands.12
- Reducing cell viability in the adrenal glands, which is associated with decreased cortisol production.12,16
- Inducing a compensatory rise in ACTH, which leads to adrenal hyperplasia. Research suggests mercury-induced adrenal hyperplasia may eventually cause adrenal atrophy and the development of Addison’s disease (adrenal insufficiency).17
- Reducing the production of cholesterol, the precursor for all steroid hormones.
- Impairing the conversion of cholesterol to pregnenolone by decreasing cytochrome P-450 activity, thus hampering the activity of 21-hydroxylase, the enzyme that converts 17-hydroxyprogesterone to 11-deoxycortisol.
- Competitively binding to sulfhydryl groups to block 17-hydroxyprogesterone, leading to increased plasma progesterone levels and adrenal androgen production.
- Converting cytochrome P-450 into the inactive P-420.12
- Inhibiting catecholamine degradation via the inactivation of S-adenosyl-methionine, which can lead to the accumulation of epinephrine and hyperhidrosis, ptyalism (hypersalivation), tachycardia, and high blood pressure.16
- Having an affinity for selenoproteins, which are required for optimal endocrine function.
- Impacting thyroid function.
- Forming complexes with glutathione and glutathione reductase leads to functional changes in their levels and a subsequent decrease in 21-hydroxylase activity and cortisol and corticosterone production.12
Research confirms mercury affects the functional interrelationship between endocrine target organs and the pituitary gland. Mercury competes with steroid hormones to bind with the same binding sites in the cytochrome enzymes, thus providing a direct mechanism for mercury to hijack the steroidogenesis pathway and impact the production of corticosteroids and other steroid hormones. Overall, mercury exposure causes cytotoxicity to the adrenal gland, alters the function of the HPA axis, and augments the steroidogenic pathways to impact corticosteroid production and the responsiveness of the adrenal glands.12
 Additional Toxic Heavy Metals that Impact the Health of the Hypothalamic-Pituitary-Adrenal (HPA) Axis
Additional Toxic Heavy Metals that Impact the Health of the Hypothalamic-Pituitary-Adrenal (HPA) Axis
More research on the effects of all toxic heavy metals on the HPA axis is needed. While we do not know as much about how other toxic metals may affect the health of the HPA axis, we do know that exposure to nickel, arsenic, or aluminum is associated with the reduced production of cortisol.17
Research also shows the methylated metabolites of inorganic arsenic reduce nuclear translocation and DNA binding and mediate the activation of glucocorticoid receptors.8
 Next Steps – Order Adrenal Stress Index and Toxic Metals & Elements Test Panels
Next Steps – Order Adrenal Stress Index and Toxic Metals & Elements Test Panels
The original Adrenal Stress Index panel is the ideal first step to assess the health of the HPA axis. If the function of the HPA axis is not optimal, then consider testing for current exposure to and the approximate stored levels of toxic heavy metals with Toxic Metals and Elements (TME) Test Panels. DiagnosTechs offers six different TME Test Panels:
- Salivary Metals and Elements (SMETALS)
- Urinary Metals and Elements (UMETALS)
- Salivary and Urinary Metals and Elements Combo Panel (CMETALS)
- Timed Salivary Metals and Elements (TSMETALS)
- Timed Urinary Metals and Elements (TUMETALS)
- Timed Salivary and Urinary Metals and Elements Combo (TCMETALS)
The regular (single collection) TME Panels primarily assess baseline current exposure levels to metals and elements rather than the stored or past exposure to metals and elements. The Timed TME Panels, on the other hand, are designed to be used in conjunction with a chelating or provoking agent, which will mobilize the stored pool of metals to assess past exposure to and stored levels of metals and elements, in addition to current exposure.
Targeted Heavy Metal Detox Protocol
Once test results reveal which toxic heavy metals are clinical concerns, an efficient and individualized treatment protocol that includes reducing exposure to and supporting the detoxification of specific toxic metals can be prescribed to support the optimal health of your patients.
 To place a test order, click here. As a reminder, DiagnosTechs will drop ship test kits directly to your patients. You may select this option at the top of the order form.
To place a test order, click here. As a reminder, DiagnosTechs will drop ship test kits directly to your patients. You may select this option at the top of the order form.
Please visit our Provider Tools page for more information about choosing the right test, TME – FAQs for Providers, provocation protocols, test result interpretation, and treatment options.
 References:
References:
- Gruszecki, A. Adrenal Fatigue: Environmentally Induced Adrenal Hypofunction? NDNR. 2020; 16(2): 1-5.
- Crinnion W, Pizzorno JE. Clinical Environmental Medicine: Identification and Natural Treatment of Diseases Caused by Common Pollutants. Louis, MO: Elsevier; 2019.
- Jomova K, Jenisova Z, Feszterova M, et al. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. 2011;31(2):95-107. doi:10.1002/jat.1649
- Lafuente A. The Hypothalamic-Pituitary-Gonadal Axis Is Target of Cadmium Toxicity. An Update of Recent Studies and Potential Therapeutic Approaches. Food Chem Toxicol.2013; 59: 395-404.
- Sepulchro Mulher LCC, Simões RP, Rossi KA, et al. In vitro cadmium exposure induces structural damage and endothelial dysfunction in female rat aorta. Biometals. 2023;36(6):1405-1420. doi:10.1007/s10534-023-00526-5
- Caride A, Fernández-Pérez B, Cabaleiro T, et al. Cadmium chronotoxicity at pituitary level: effects on plasma ACTH, GH, and TSH daily pattern. J Physiol Biochem. 2010;66(3):213-220. doi:10.1007/s13105-010-0027-5
- Caudle M. This can’t be stressed enough: The contribution of select environmental toxicants to disruption of the stress circuitry and response. Physiol Behav. 2016; 166: 65–75.
- Liu D, Shi Q, Liu C, et al. Effects of Endocrine-Disrupting Heavy Metals on Human Health. Toxics. 2023;11(4):322. doi:10.3390/toxics11040322
- Schreier H, Hsu H, Amarasiriwardena C, et al. Mercury and psychosocial stress exposure interact to predict maternal diurnal cortisol during pregnancy. Environ Health. 2015; 14: 28.
- Virgolini MB, Chen K, Weston DD, et al. Interactions of chronic lead exposure and intermittent stress: consequences for brain catecholamine systems and associated behaviors and HPA axis function. Toxicol Sci. 2005;87(2):469-482. doi:10.1093/toxsci/kfi269
- Rattan S, Zhou C, Chiang C, et al. Exposure to endocrine disruptors during adulthood: consequences for female fertility. J Endocrinol. 2017;233(3):R109-R129. doi:10.1530/JOE-17-0023
- Tan SW, Meiller JC, Mahaffey KR. The endocrine effects of mercury in humans and wildlife. Crit Rev Toxicol. 2009;39(3):228-269. doi:10.1080/10408440802233259
- Rana, S. Perspectives in endocrine toxicity of heavy metals – A review.Biol Trace Elem Res. 2014; 160(1): 1-14. doi:10.1007/s12011-014-0023-7
- Schreier HM, Hsu HH, Amarasiriwardena C, et al. Mercury and psychosocial stress exposure interact to predict maternal diurnal cortisol during pregnancy. Environ Health. 2015;14:28. doi:10.1186/s12940-015-0016-9
- Laks DR. Luteinizing hormone provides a causal mechanism for mercury associated disease. Med Hypotheses. 2010;74(4):698-701. doi:10.1016/j.mehy.2009.10.036
- Rice KM, Walker EM Jr, Wu M, et al. Environmental mercury and its toxic effects. J Prev Med Public Health. 2014;47(2):74-83. doi:10.3961/jpmph.2014.47.2.74
- Perez-Cadahia B, Laffon B, Porta M, et al. Relationship between blood concentrations of heavy metals and cytogenetic and endocrine parameters among subjects involved in cleaning coastal areas affected by the ‘Prestige’ tanker oil spill. Chemosphere. 2008; 71(3): 447-55.